The journey of health technology to acquire any market share has many uncertainties, and thus the failure rate of start-ups is relatively high. For example, a trade press article (Chase, 2016) indicates that 98% of digital health start-ups are almost dead. For various reasons, their products do not manage to reach the market and gain any traction.
A consortium of partners in the COVID-X project has been trying to accelerate one of the hardest entrepreneurial challenges Europe and other places worldwide face over the course of almost two years. It is launching a health tech start-up in the market. In parallel to accelerating 28 products that help to solve challenges caused by COVID-19, CIVITTA has analysed the ecosystem and has created a list of recommendations regarding the legal challenges.
The recommendations are primarily beneficial to the innovators trying to navigate the landscape of launching a product to market and policymakers who can aid in solving the bottlenecks. Nevertheless, the healthcare institutions would find the recommendations helpful to be more aware of the challenges the innovators face and could work on breaking down the barriers from their side.
Products and services in the healthcare industry significantly differ from innovation in any other sector. In contrast to the default approach widely favoured by tech start-ups to “fail fast and break things,” the healthcare industry is not so forgiving since the users of solutions will probably be patients whose safety is paramount and physicians. As a result, a slower, more deliberate route to market and reimbursement has been more likely to succeed. Nevertheless, creating a good product in the healthcare sector is only a small portion of the overall success of the technology, even to have a chance to start making an impact on the end-users identified in the traditional business design workshops.
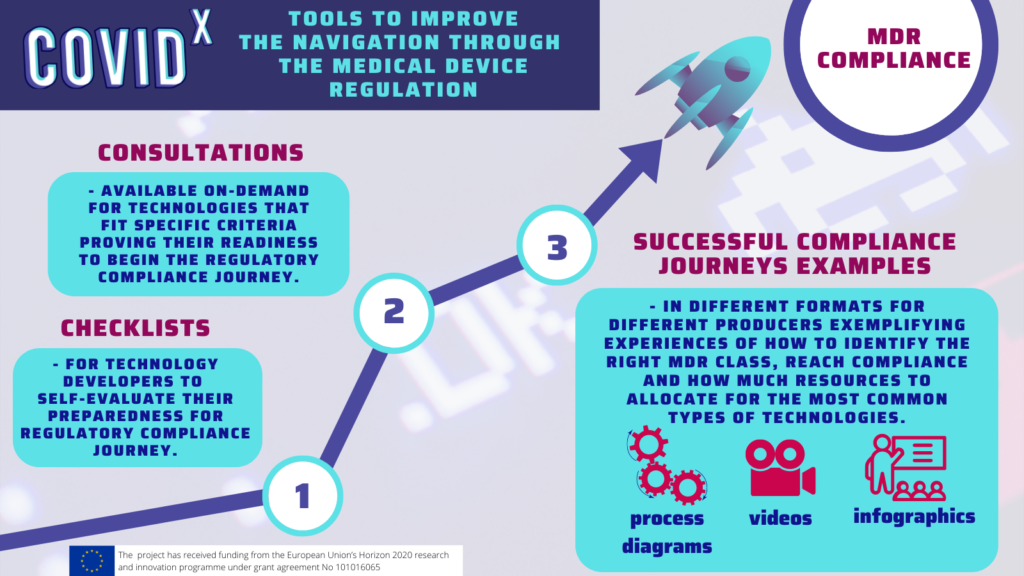
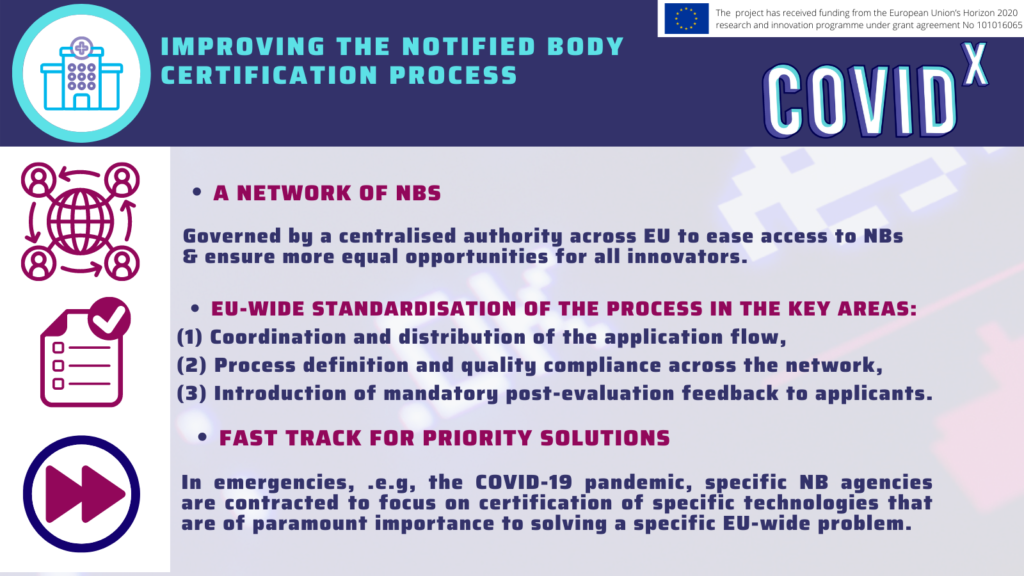
From a range of the challenges the innovators face, the key challenges that were identified in the regulatory domain were associated with the understanding and interpretation of the Medical Device Regulation, the shortage of Notified Bodies as well as the fragmentation of the local regulations in terms of Health Technology Assessment and reimbursement paths.
The identified challenges are hindering a fast uptake of digital solutions, causing disparities in the overall uptake of novel solutions between the member states and putting SMEs at a disadvantage compared to large enterprises in terms of quickly bringing novel solutions to market. To learn more about what each stakeholder can do to bring more innovations to the healthcare sector, please join the COVID-X final conference “Back to the Future”, Area42, Brussels. In the meantime, you can read the full report covering the legal and ethical challenges faced by the innovators participating in the COVID-X project and potential solutions to help them.