Find out how MedTech legal obstacles can be solved by reading Recommendations for Healthcare Authorities!
Digitalisation is a clear path and destination in the 21st-century healthcare industry. The need for digital solutions in healthcare has been recognised long before the emergence of COVID-19. However, the pandemic might have arguably given the final push the healthcare industry needed to adopt digital solutions for facilitating prognosis, diagnosis, treatment and after-care monitoring.
In the last two years, the European MedTech market has been growing rapidly, with more than 33 000 medical technology companies based in Europe, according to MedTech Europe 2021 research. Thousands of new digital health solutions are emerging on the market and thousands more are being developed, mainly by start-ups and tech companies entering the sector for the first time. However, the expert estimations are quite discouraging for the progress of the start-up landscape. Dave Chase, a Digital Health expert, states a particularly concerning view that nearly 98% of digital health start-ups are the walking dead right now. So, the question is, what is hindering the progress of the health tech innovators? This problem is predominantly rooted in the complex nature of medical devices’ regulations. Regulatory compliance of the new digital solutions requires time (up to several years) and financial resources and, right now, the MedTech market is progressing much faster than the policy makers are being able to adapt to its pace.
Many experts focused on researching the current situation to provide key solutions. As a Horizon 2020 project dedicated to narrowing down the divide between technology breakthroughs and the healthcare system through the power of data, COVID-X has also joined the action. In fact, the document Recommendations for Healthcare Authorities produced by the consortium partner CIVITTA, represents the result of our efforts. By combining the analysis of the relevant literature with the experience of the data-driven start-ups participating in the COVID-X programme, the document pinpointed alarming issues hindering the uptake of novel digital and data-driven solutions.
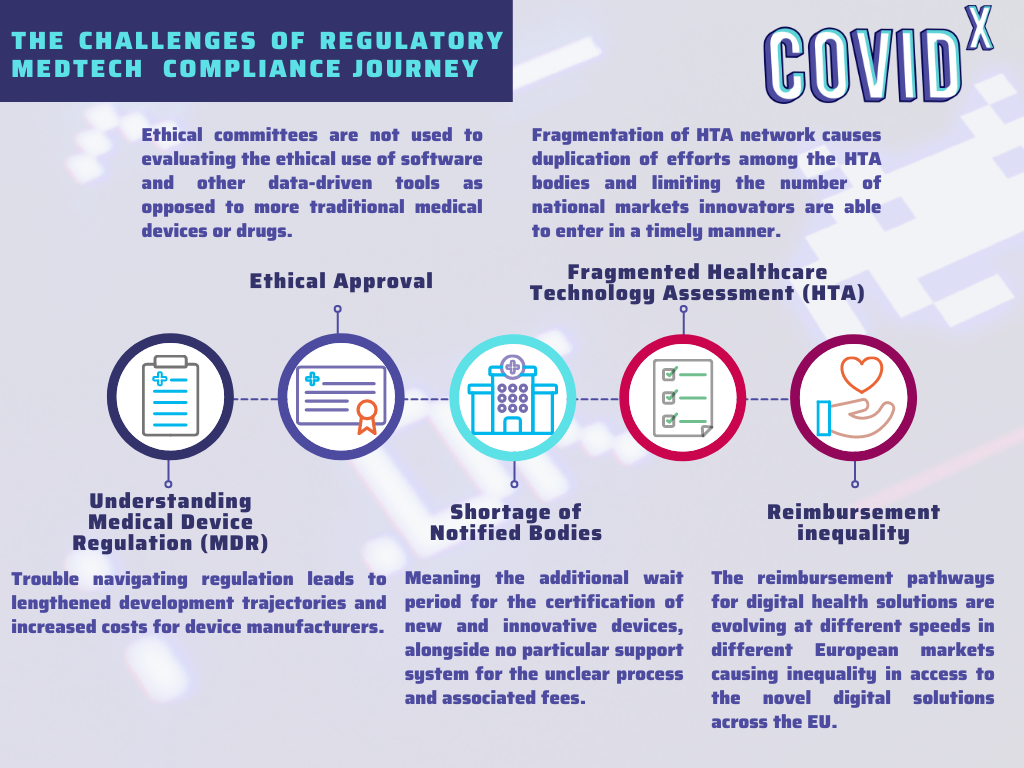
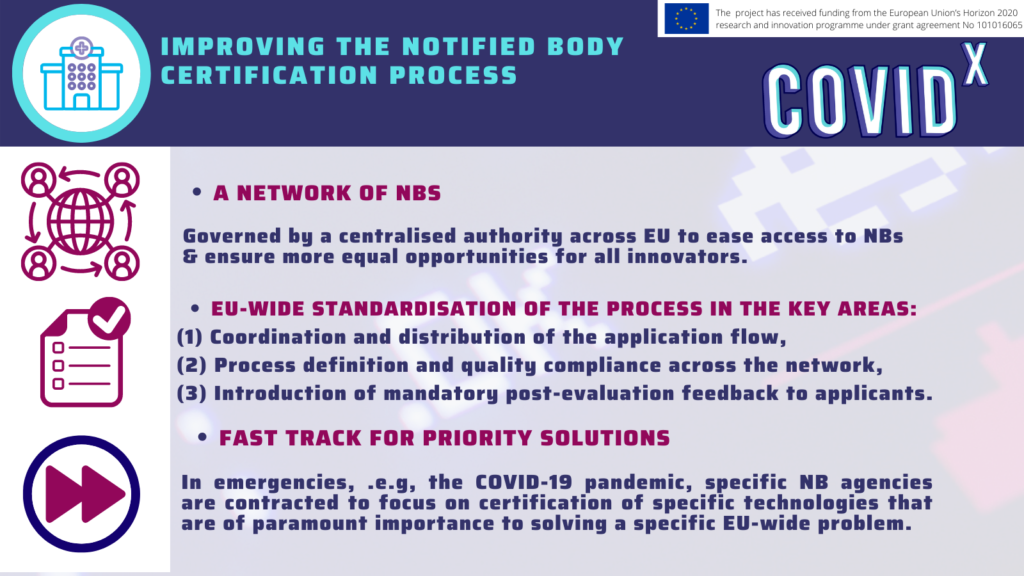
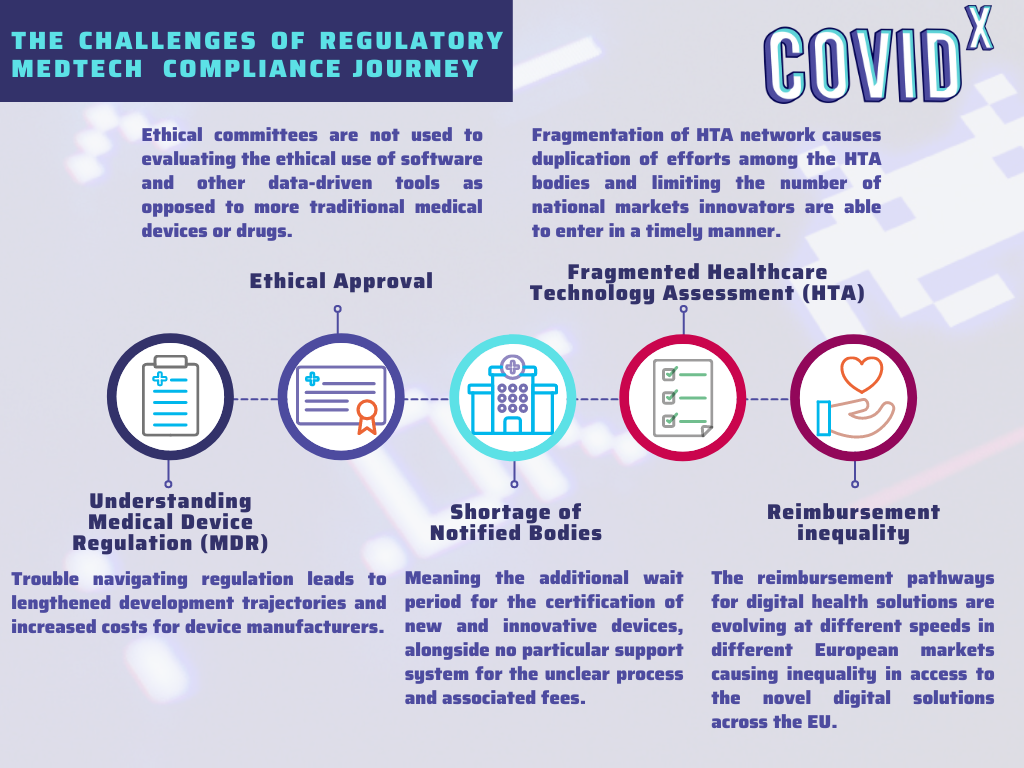
The key challenges identified in the regulatory domain were associated with the understanding and interpretation of the Medical Device Regulation, the shortage of Notified Bodies, and the fragmentation of the local regulations in terms of Health Technology Assessment and reimbursement paths. The graphic down below summarises the main obstacles of the regulatory Medtech journey.
The identified challenges are causing a slow uptake of digital solutions, the disparities in the overall uptake of novel solutions between the member states and are putting SMEs at a disadvantage compared to large enterprises in terms of quickly bringing novel solutions to market.
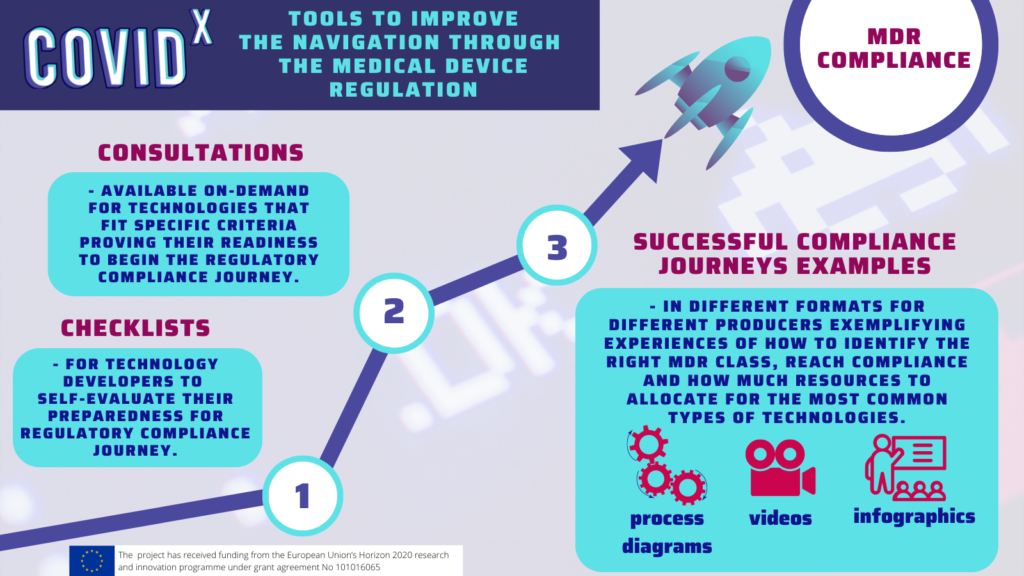
But is the future of MedTech beyond the state of salvation? Can the countless medical innovations be saved from the digital “Death Valley” they have been facing? We believe that they can! Our deliverable represents a summarised action plan for policymakers on a national as well as European level with advice on future steps to facilitate the implementation of novel medical solutions. Therefore, stay tuned as we explore the key COVID-X recommendations for each health tech legal and regulatory framework stage!
To read our recommendations for healthcare authorities, click here.